In 1905, Einstein’s comparison between dim (or dilute) light, and an ideal (dilute) gas led him to conclude that under certain conditions, light will behave as a particle.
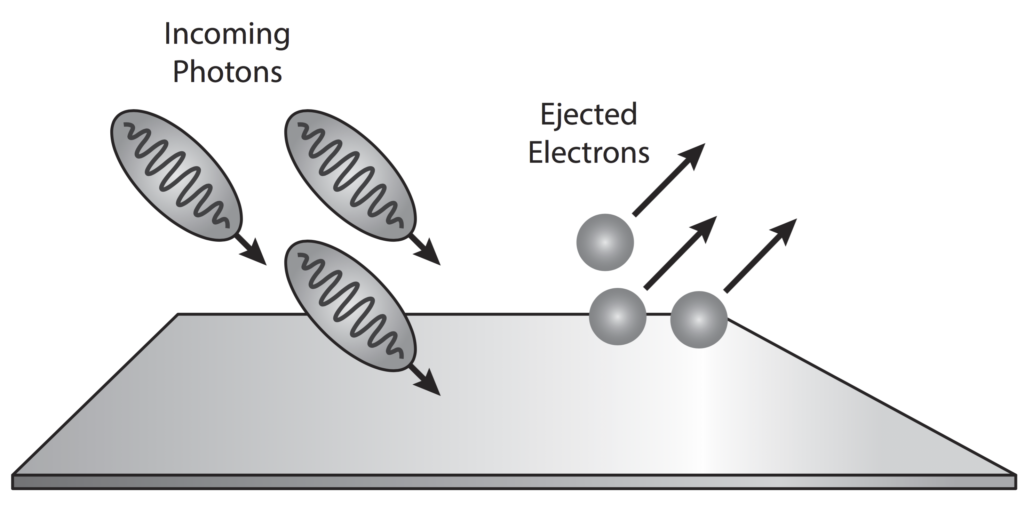
The photoelectric effect is a result of electron-photon pair collisions.
“[Planck’s] derivation was of unparalleled boldness, but found brilliant confirmation. … However, it remained unsatisfactory that the [classical mechanical] analysis, which led to [Planck’s Radiation Law], is incompatible with quantum theory, and it is not surprising that Planck himself and all theoreticians who work on this topic incessantly tried to modify the theory such as to base it on noncontradictory foundations.” –Einstein
In 1887 Hertz confirmed the wave nature of light and also (inadvertently) its particle (photon) nature as well.
In 1905, at the age of 26, Einstein published four major papers and finished his PhD thesis. Each of these papers was groundbreaking and would change physics forever.
With his work in 1916-7, Einstein was able to arrive at a “much more” quantum derivation of Planck’s Radiation Law. However, in the end he fell short, having to rely on assumptions. In 1924, Satyendra Nath Bose provided the first “fully” quantum derivation of Planck’s radiation law, which revealed the deeper nature of light that had eluded everyone else, including Einstein. With this work, he created the new area of physics known as quantum statistics.
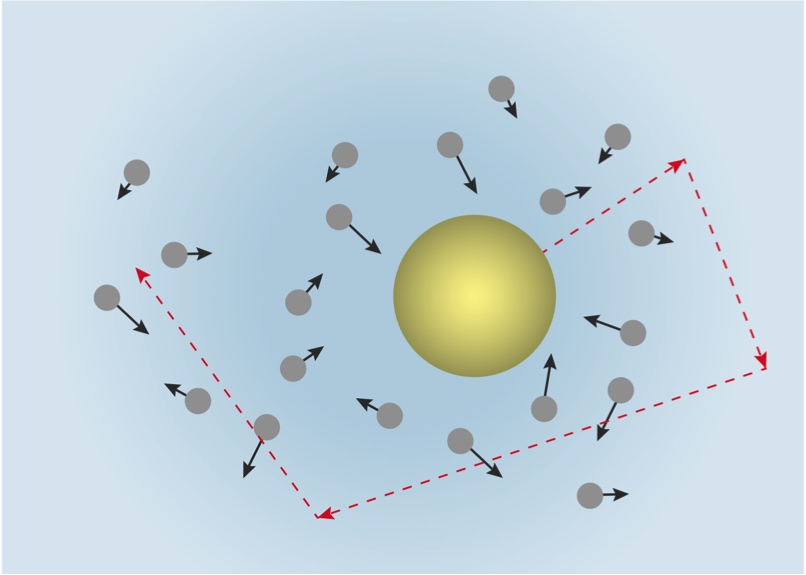
In 1905, a young Albert Einstein wrote a paper (while working as a patent clerk) on a well-known physical phenomenon of the time, Brownian motion, which had first been noted in 1827. Einstein’s theory correctly described Brownian motion, and at the heart of his theory was the existence of atoms that were in constant motion.