In 1905, Einstein’s comparison between dim (or dilute) light, and an ideal (dilute) gas led him to conclude that under certain conditions, light will behave as a particle.
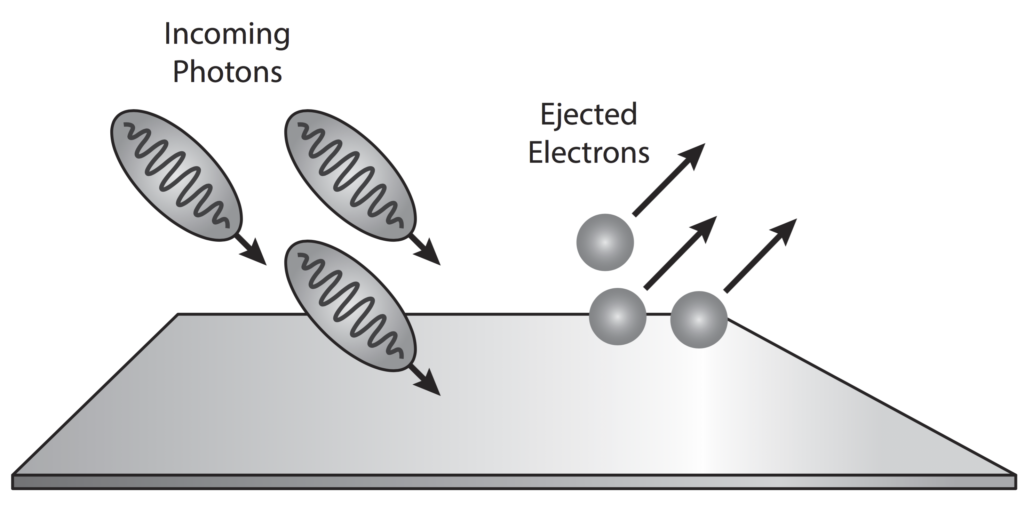
The photoelectric effect is a result of electron-photon pair collisions.
“[Planck’s] derivation was of unparalleled boldness, but found brilliant confirmation. … However, it remained unsatisfactory that the [classical mechanical] analysis, which led to [Planck’s Radiation Law], is incompatible with quantum theory, and it is not surprising that Planck himself and all theoreticians who work on this topic incessantly tried to modify the theory such as to base it on noncontradictory foundations.” –Einstein
In 1887 Hertz confirmed the wave nature of light and also (inadvertently) its particle (photon) nature as well.
The Problem with Schrödinger’s Wave Equation
The biggest question still plaguing Schrödinger’s wave equation was the role of the wavefunction. Sure, mathematically it’s clear: it’s the solution to Schrödinger’s wave equation and the “all-powerful function” as a result. However, physically it was still a big mystery to everyone, including Schrödinger himself.
With his work in 1916-7, Einstein was able to arrive at a “much more” quantum derivation of Planck’s Radiation Law. However, in the end he fell short, having to rely on assumptions. In 1924, Satyendra Nath Bose provided the first “fully” quantum derivation of Planck’s radiation law, which revealed the deeper nature of light that had eluded everyone else, including Einstein. With this work, he created the new area of physics known as quantum statistics.
Einstein Revisits His Theory of Light
By 1911, Einstein had already hypothesized that light consists of particles he called light quanta (later called photons). Moreover, he had shown that light has an inherent quality, whereby it exhibits both wave and particle properties. Although, he had seen further than anyone into the mysterious nature of light, it continued to perplex him:
“I do not ask anymore whether these [light] quanta really exist. Nor do I attempt any longer to construct them, since I now know my brain is incapable of advancing in that direction.”
In 1921, Einstein won the Nobel Prize. The citation reads:
“To Albert Einstein for his services to theoretical physics and especially for his discovery of the law of the photoelectric effect.”
To be sure, Einstein is being acknowledge for the “the law of the photoelectric effect”, in other words for his photoelectric equation, but not for the photon concept. This attitude would persist until 1923, when new experimental results would convert pretty much everyone to the existence of photons.
Niels Bohr’s Early Career
In March of 1912 Niels Bohr (1885–1962) arrived in Manchester to begin working with Rutherford. Previously, he had worked with Thomson in Cambridge. Unfortunately, their relationship had been strained from the start, and never really flourished as Bohr had hoped. Writing to his brother Harald, Bohr said:
“… Thomson has so far not been as easy to deal with as I thought the first day. …”
Perhaps, Bohr’s initial encounter with Thomson was to blame, where upon entering Thomson’s office, Bohr proclaimed:
“This is wrong.”
Max Planck’s Early Life
Max Planck (1858–1947) was born in Kiel, (in modern day Germany), the sixth child to the distinguished jurist and professor of law at the University of Kiel, Johann Julius Wilhelm Planck and his second wife, Emma Patzig. His family culture would bestow in Planck’s life and work a sense of excellence in scholarship, incorruptibility, idealism, reliability, and generosity.
In 1867, when Planck was nine, his father received an appointment at the University of Munich. The family moved and Planck enrolled in the city’s Maximilian Gymnasium where his interest in physics and mathematics was piqued. However, Planck excelled in his other studies as well, in particular music. Thus at the time of graduation, now 16, Planck had the difficult decision of choosing a future in either music or physics; he chose physics.
Continue …
The transition from quantum theory to quantum mechanics began in 1926 with work of Erwin Schrödinger.
The zero point energy of a system is a direct consequence of the Heisenberg uncertainty principle.
Quantum probability is a natural consequence of Schrödinger’s formulation; Heisenberg’s includes it explicitly.
In terms of quantum theory, increasing the intensity of light means increasing the number of photons.
In 1923, Arthur Compton’s (1892-1962) light scattering experiments provided further support for Einstein’s hypothesis for the particle nature of light.
Although possible, helium does not easily solidify due to quantum effects related to its small atomic size.
The probabilistic interpretation of quantum mechanics is due to Max Born.
Einstein’s light quanta hypothesis met with tremendous resistance, taking almost twenty years after its introduction in 1905 to be fully accepted. Despite such opposition, Einstein continued to use the concept in his work with significant success.
In 1905, Einstein established wave-particle duality for light. In 1923, de Broglie extended it to all quantum particles. In an interview in 1963 de Broglie reflected on his epiphany:
“As in my conversations with my brother we always arrived at the conclusion that in the case of X-rays one had both waves and [particles], thus suddenly – … it was certain in the course of summer 1923 – I got the idea that one had to extend this duality to material particles, especially to electrons.”
In 1925, Einstein predicted a very unusual phase transition that occurs for the quantum ideal gas. Einstein describes the phenomenon in a letter to Paul Ehrenfest (1880–1933):
“From a certain temperature on, the molecules ‘condense’ without attractive forces, that is, they accumulate at zero velocity.”
In other words, as the temperature is lowered, the atoms in the gas begin to “pile up” or condense into the lowest (single particle) energy state, which is the one with zero kinetic energy; there’s a critical temperature whereby a phase transition (now called (Bose-Einstein condensation) occurs. This effect becomes most pronounced as the temperature is lowered to absolute zero, at which point, all the gas atoms condense into this lowest energy state. This phenomenon is an example of quantum entanglement.
Einstein predicted a new phase transition for matter (Bose-Einstein Condensation) in 1925; it took until 1995 to verify it experimentally.
Einstein predicted stimulated emission can occur when light and matter interact; this idea forms the basis of the modern laser.
“The great initial success of the quantum theory cannot convert me to believe in that fundamental game of dice.” –Einstein (1944)
“If all this damned quantum jumping were really here to stay, I should be sorry I ever got involved with quantum theory.” –Schrödinger
Einstein’s 1916-7 approach was as close as anyone got to a full quantum derivation of Planck’s Radiation Law, until an unknown physicist from Calcutta, India revisited the problem in 1924, and created the area of quantum statistics.
In 1925, Einstein made his last contribution to quantum theory (consider by many to be his last significant scientific contribution as well) with his work on the quantum ideal gas.
After 1925, Einstein turned his back forever on quantum mechanics, arguing that its probabilistic nature was a fundamental flaw.
If in 1917 Einstein truly saw the probabilistic nature as a shortcoming, later he would be less forgiving.
Einstein’s 1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light is often (wrongly) referred to as his paper on the photoelectric effect.
In his1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light, Einstein showed how his newly introduced light quanta hypothesis could be used to interpret several well-known experimental observations, the most notable of these phenomena being the photoelectric effect.
The major theme of Einstein’s 1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light, was that light (under certain circumstances) behaves as if it’s comprised of individual particles rather than waves. These particles, or “chunks” of light were originally called light quanta, and then later came to be called photons.
It was the first of Einstein’s 1905 papers, On a Heuristic Point of View Concerning the Production and Transformation of Light, which he referred to as “very revolutionary” – the only time he would ever say this about any of his work, in fact – and which, in part would win him the Nobel Prize in 1921.
At the atomic level, energy occurs as “chunks” or quanta.
Transitions between the energy states of atoms and molecules require a specific amount of energy, nothing more, nothing less; it’s only these particular values that are allowed by nature.
The energy states available to atoms and molecules occur at specific intervals. In other words, they are discrete rather than continuous.
Quantum Theory was born in 1900 with the pioneering work of German physicist Max Planck (1858-1947).