In 1905, Einstein’s comparison between dim (or dilute) light, and an ideal (dilute) gas led him to conclude that under certain conditions, light will behave as a particle.
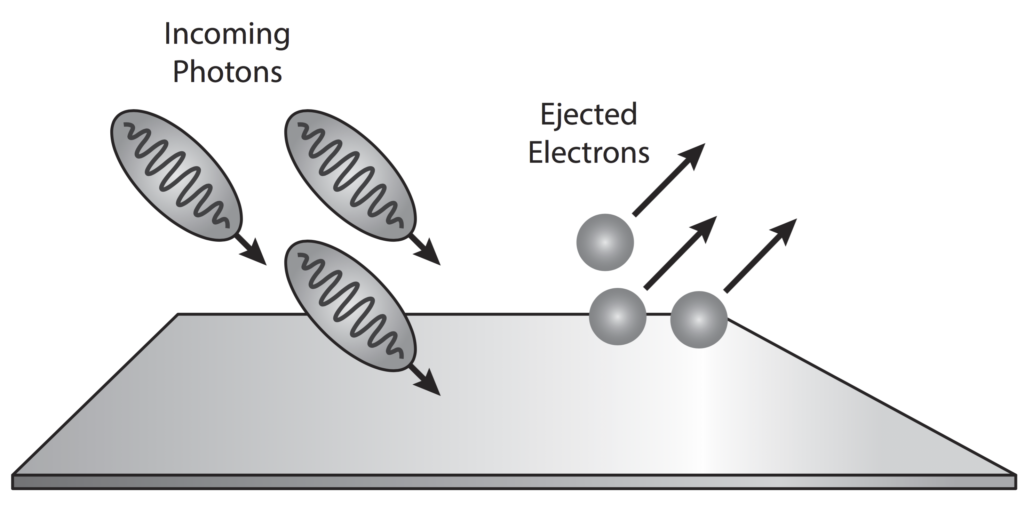
The photoelectric effect is a result of electron-photon pair collisions.
In 1887 Hertz confirmed the wave nature of light and also (inadvertently) its particle (photon) nature as well.
Einstein Revisits His Theory of Light
By 1911, Einstein had already hypothesized that light consists of particles he called light quanta (later called photons). Moreover, he had shown that light has an inherent quality, whereby it exhibits both wave and particle properties. Although, he had seen further than anyone into the mysterious nature of light, it continued to perplex him:
“I do not ask anymore whether these [light] quanta really exist. Nor do I attempt any longer to construct them, since I now know my brain is incapable of advancing in that direction.”
In 1921, Einstein won the Nobel Prize. The citation reads:
“To Albert Einstein for his services to theoretical physics and especially for his discovery of the law of the photoelectric effect.”
To be sure, Einstein is being acknowledge for the “the law of the photoelectric effect”, in other words for his photoelectric equation, but not for the photon concept. This attitude would persist until 1923, when new experimental results would convert pretty much everyone to the existence of photons.
In terms of quantum theory, increasing the intensity of light means increasing the number of photons.
In 1923, Arthur Compton’s (1892-1962) light scattering experiments provided further support for Einstein’s hypothesis for the particle nature of light.
Shorter wavelength blue light is scattered more by air molecules than red light. It’s this “Rayleigh scattering” that results in the blue color of the sky.
Einstein’s light quanta hypothesis met with tremendous resistance, taking almost twenty years after its introduction in 1905 to be fully accepted. Despite such opposition, Einstein continued to use the concept in his work with significant success.
If the wavelength of visible light was as large as that of a sound wave you’d be able to see around corners.
Newton felt there were some properties a wave theory of light simply could not explain, such as diffraction. For example, diffraction is the property of waves that allow them to bend around objects and spread through openings. In the case of sound waves, it’s why a sound in one room can often be heard in another room farther away by someone not directly in the path of the oncoming sound; the sound wave travels from the one room, spreading out through the doorway into the other room where it’s then heard. Light doesn’t appear to behave this way, after all, you can’t see around corners – can you? The diffraction of light is usually unnoticeable because light waves have such short wavelengths – much smaller compared to the wavelengths of sound waves. Nonetheless, light will diffract if the opening is small enough.
In 1905, Einstein established wave-particle duality for light. In 1923, de Broglie extended it to all quantum particles. In an interview in 1963 de Broglie reflected on his epiphany:
“As in my conversations with my brother we always arrived at the conclusion that in the case of X-rays one had both waves and [particles], thus suddenly – … it was certain in the course of summer 1923 – I got the idea that one had to extend this duality to material particles, especially to electrons.”
The speed of light was measured as early as 1862 by Leon Foucault giving good agreement with the modern value of 299,792.458 Km/s.
In 1905, Einstein said light is a particle (photon) with energy proportional to its frequency. Although photon momentum was known much earlier to Einstein, he waited until 1916 to finally declare it.
By the end of the eighteenth century, heat along with its cohorts light, magnetism and electricity were regarded as an imponderable fluid capable of flowing between the spaces assumed to be present in matter.
In 1789, Lavoisier published An Elementary Treatise on Chemistry where he describes 33 elements. The list begins with caloric and continues with light, oxygen, nitrogen and hydrogen.
In 1666, Newton bought his first prism with the motivation of disproving Descartes wave theory of light. In 1672, he gave a brief account of his findings, in the form of a letter, to the Royal Society, whereby – after a bit of convincing – it was published in the Philosophical Transactions of the Royal Society. Although not unanimous, Newton’s work met with much praise. However, one critic’s words would resound with Newton, thus beginning a lifetime feud.
Robert Hooke (1635–1703), who was considered the expert on the subject in England, sent a lengthy critique. In short, it pretty much said Hooke had performed all the same experiments, drawn different conclusions, and that Newton was outright wrong. In 1704, Newton finally published a full account of his theory of light in Opticks. To be sure, Newton had already drafted a treatise covering much of this work by 1672,
Einstein’s 1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light is often (wrongly) referred to as his paper on the photoelectric effect.
In his1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light, Einstein showed how his newly introduced light quanta hypothesis could be used to interpret several well-known experimental observations, the most notable of these phenomena being the photoelectric effect.
The major theme of Einstein’s 1905 paper, On a Heuristic Point of View Concerning the Production and Transformation of Light, was that light (under certain circumstances) behaves as if it’s comprised of individual particles rather than waves. These particles, or “chunks” of light were originally called light quanta, and then later came to be called photons.
It was the first of Einstein’s 1905 papers, On a Heuristic Point of View Concerning the Production and Transformation of Light, which he referred to as “very revolutionary” – the only time he would ever say this about any of his work, in fact – and which, in part would win him the Nobel Prize in 1921.