Ludwig Boltzmann‘s Early Career
Ludwig Boltzmann (1844–1906) was born in Vienna and attended the University of Vienna, where he received his doctorate in 1867. Boltzmann was a restless spirit, changing (by choice) from one academic position to another, a total of seven times in his almost forty-year career.
From the early 1870s on, Boltzmann was a scientific superstar and very much in demand. To get Boltzmann to accept a professorship of theoretical physics at the University of Vienna in 1894, the Austrian minister of culture had to offer him the highest salary then paid to any Austrian university professor. Boltzmann had already been a professor at the university of Vienna twice before, once from 1867–1869 as an assistant professor, and a second time from 1873–1876 as a professor of mathematics. Nonetheless, in 1900 he left for a third time.
His final return to Vienna was in 1902, upon which he succeeded, none other than himself as professor of theoretical physics; the position had been vacant since his departure two years prior. Well aware of his wandering tendencies, the Austrian authorities allowed Boltzmann to return conditional upon his promise never to take another job outside of Austria; a promise he kept. Boltzmann’s personality was such that he would fluctuate between extreme highs and lows, and he himself joked about this duality in behavior, likening it to the fact that he was born between Shrove Tuesday and Ash Wednesday – between celebration and penance, so to speak. Today Boltzmann would most likely be diagnosed as bipolar (he had other health issues: asthma, migraines, poor eyesight, and angina pains).
Boltzmann and The Second Law
In 1866, at the age of twenty-two, Boltzmann wrote his first paper of any significance entitled On the Mechanical Meaning of the Second Law. Here he contrasted the ambiguous nature of the second law against the very secure stature of the conservation of energy described by the first law. He attempted to provide a general proof of the second law and relate it to classical mechanics as described by Newton.
Although Maxwell didn’t formulate a theory of the second law himself, he regarded this approach as fundamentally misguided since for him the second law was purely probabilistic or statistical in nature, and therefore had no basis in a purely classical mechanical description. Boltzmann was not alone in his misdirection, Clausius also attempted to bring the second law to terms with the principles of mechanics. Boltzmann soon realized this approach was fatally flawed, whereas Clausius, even after several attempts, was never convinced.
Microstates
Recall, that the Maxwell distribution describes the range, or distribution of speeds for the atoms comprising an ideal gas in equilibrium at a given temperature. We have already learned that “energy of motion” is kinetic energy, and clearly, something that has a speed must be in motion and consequently have kinetic energy. Therefore, the Maxwell distribution is also describing the kinetic energy distribution of the ideal gas atoms at equilibrium, or to put it another way: the kinetic energy distribution of the entire system of an ideal gas at equilibrium is described by the Maxwell distribution; this is what Boltzmann realized.
In 1868, Boltzmann was then able to show that the idea of a distribution could be extended to the total energy of a system at equilibrium, both the kinetic and the potential energy contributions. In fact, with his more general approach to the problem, Boltzmann was also able to obtain the Maxwell distribution for a system of ideal gas atoms at equilibrium.
So, while the gas atoms of a given system are zipping around, colliding with each other, and the walls of the container that hold them, when the system is at equilibrium, the system as a whole is sampling a range of total energies given by the Boltzmann distribution. Each of these energies describes the system as being in a certain microstate. Specifically, a single microstate is described by all the positions of the gas atoms and their respective velocities at a given instant in time. As time goes by, the system will move from one microstate to another and given enough time, the system will sample all the microstates available to it. However, it will not sample each microstate with the same frequency; it will sample the more probable microstates more often. According to the Boltzmann distribution, the microstates with a lower total energy are the more probable ones, with the probability of their occurrence given by the Boltzmann probability.
Entropy and Probability
The Boltzmann distribution, the Boltzmann probability, and microstates are fundamental ideas of statistical mechanics, which allow us to correctly calculate certain things about a system. And not just a system of gas atoms, but for all classical (as oppose to quantum) systems. Things such as pressure and temperature can be calculated – sometimes with the help of computer simulations. So, while we can’t see atoms, their crazy motions or the microstates that result, with the methods developed by Boltzmann and others around these concepts, we can correctly describe many of the things that we observe in our daily lives.
When you look at something, what you’re seeing is its physical state, or macrostate. Again, consider a balloon filled with air. You can’t see the air molecules moving around as they collide with each other, and the walls of the balloon. What you can see is the shape (volume) of the balloon, and you can measure the temperature. In this case, the macrostate of your system is well described by properties you can see and measure: temperature and volume. However, the microstates, resulting from the colliding molecules, are hidden from view.
So, in the end, the many microstates are the hidden states of the system, while the single macrostate is the overall state with the physical properties we see and/or measure. In some sense, the macrostate is the course-grained, or fuzzy version of the system.
It’s kind of like this: when I was kid, I liked shaking the boxes containing my Christmas presents. As I shook a box, the contents inside would jiggle around (sometimes break) and, in essence, move from one “microstate” to another. Now, I could never see all this jiggling around (and all the different “microstates”) but what I could see in the midst of all the shaking – and what never changed – was the nicely wrapped box with the bow on top; the “macrostate” was always the same even though the “microstates” inside the box were changing with each shake.
The distinction between a macrostate, and all the microstates comprising it leads to a more fundamental understanding of entropy; more than just as “heat over temperature”. Boltzmann showed us that the more microstates a system has available to it, the higher its entropy. Recall that a spontaneous process occurs without any outside help, or work input; it just happens. Clausius taught us a spontaneous process occurs because it’s entropically favored; this is the direction that leads to an increase in entropy and is therefore the favored direction.
Using Boltzmann’s concept of microstates, we can also say: a spontaneous process occurs because this is the direction that leads to more microstates. The quintessential example of such a process may be mixing. Consider what happens when cream is poured into coffee: it spontaneously mixes. We may have imagined the cream huddling itself at the top of the cup, never fully mixing throughout. However, the second law assures us that the natural tendency will be for the coffee and cream to increase their overall entropy.
Thus, rather than huddling at the top of the cup, the cream moves through the coffee. This process of diffusion gives the cream access to much more space within the cup, than it would have otherwise had by simply remaining at the top. Moreover, the space at the top of the cup, previously occupied exclusively by the cream, is now also available to the coffee. The process of mixing has afforded both the cream and the coffee access to more available space within the cup. In this case, more space for both means more microstates overall, resulting in the entropy of the system being maximized.
So, does this mean that you will never see the creamer spontaneously unmix? Well, basically it does. The reason for this is that there’s essentially only one microstate where the cream and coffee are fully unmixed, compared to the many more microstates where they are (at least to some extent) mixed. In the end, it has to do with probability: having more ways for one thing to occur (mixing) than another thing to occur (unmixing) favors the one with the most chances. In this case, there are more chances to find the cream mixed with the coffee than there are to find it unmixed (just like there are so many more ways for you to lose the lottery than to win).
Now, Boltzmann never said there was no chance. To be sure, there’s a nonzero chance that one day the coffee and cream will end up unmixed. But the chance is so small because, once again there are so many more ways for them to be mixed. So, as the atoms comprising the coffee and creamer zip around, colliding with each other and the cup, they spend most of their time in microstates, which result in the overall mixed macrostate (physical state) we see.
An Untimely End
During the summer of 1905 Boltzmann gave a series of lectures at the University of California, Berkeley. He was at the height of his fame. Students packed in to see his lectures and colleagues sought his scientific advice. Upon his return to Vienna, (unaware of the vindicating paper written by Einstein in 1905) Boltzmann recounted his journey in a piece called A German Professor’s Journey into Eldorado.
In early 1906, Boltzmann was on vacation with his wife and daughter near the Italian seaside town of Trieste. Once again struggling with depression, Boltzmann tied a short rope to a window crossbar and hung himself, while the women were off swimming. His daughter returned to find him dead. Ludwig Boltzmann is buried in the Central Cemetery of his native Vienna. On his tombstone is Boltzmann’s equation for entropy as it relates to the number of microstates of a system:
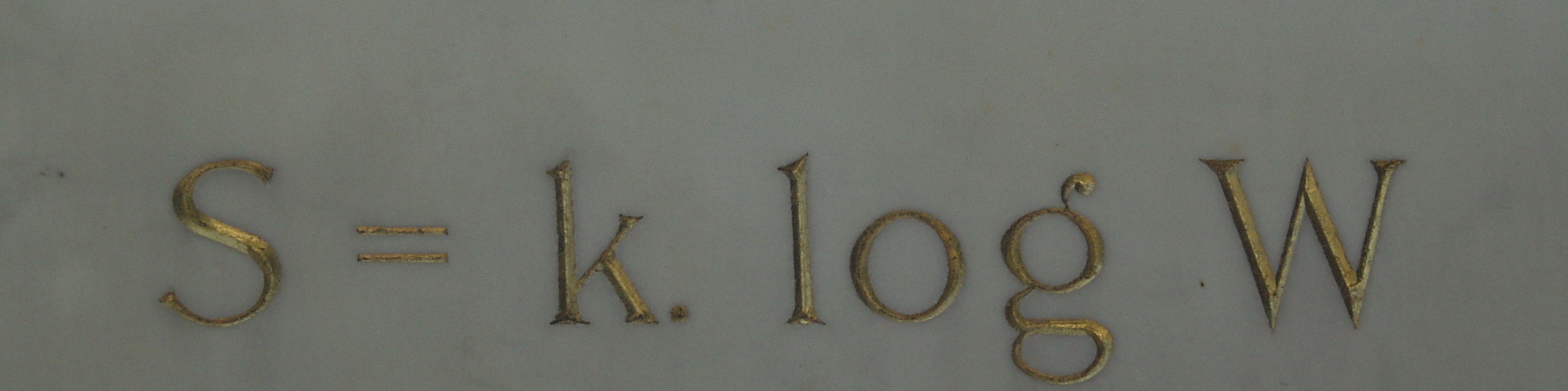
where S is the total entropy of the system, k is Boltzmann’s constant, and W is the total number of microstates corresponding to a given macrostate of the system.